In a breakthrough for renewable energy, scientists at Texas Materials Institute have uncovered a pivotal step in the oxygen reduction reaction (ORR) that is fundamental to various clean energy technologies.
The key lies in a catalyst featuring isolated iron atoms embedded in nitrogen-doped graphene, known as Fe–N–C. Despite its promise, a major stumbling block has been the unknown rate-limiting step in the ORR on Fe–N–C, hindering our ability to fully comprehend and optimize the process.
Researchers at Texas Materials Institute in Dr. Yuanyue Liu's lab: Saerom Yu, Zachary Levell, Xunhua Zhao and Zhou Jiang, have now tackled this challenge by employing state-of-the-art molecular dynamics simulations, calculating activation energies for each step under a constant electrode potential. Contrary to the prevailing belief that a hydrogenation step imposes a bottleneck on the reaction rate, the team's findings indicate that the rate-limiting step involves the replacement of an adsorbed water molecule with an oxygen molecule on the iron catalyst. Notably, this exchange occurs through a coordinated movement that avoids leaving the catalytic site bare.
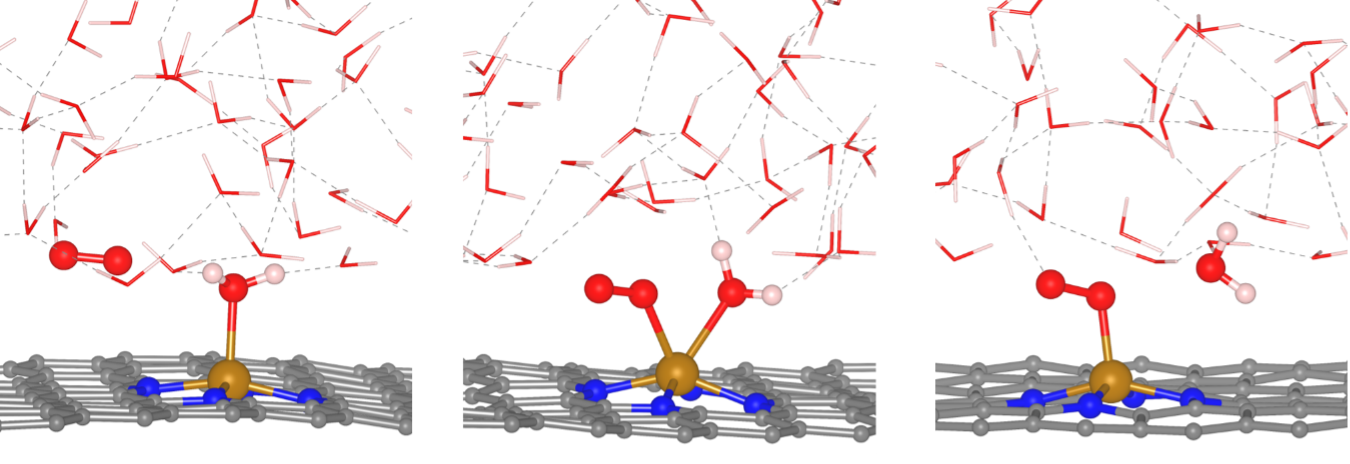
The rate-limiting step of oxygen reduction reaction on single atom catalyst is replacing water molecule by oxygen molecule.
The significance of this discovery extends beyond the laboratory. Although the identified step appears to be a "thermal" process—often considered potential-independent—a remarkable observation emerged. The barrier to this critical step diminishes as the electrode potential decreases. This unexpected behavior can be explained by stronger binding between iron and oxygen and weaker binding between iron and water at lower potentials. The dynamic interplay arises from oxygen gaining electrons and water donating electrons to the catalyst. It also suggests designing catalytic sites with more negative charges would benefit the reaction.
In essence, this study not only deepens our understanding of the ORR on Fe–N–C but also underscores the importance of delving into the kinetics of heterogeneous electrochemistry. The implications could pave the way for more efficient and sustainable energy technologies on a broader scale.
Dr. Yuanyue Liu, is an Assistant Professor in the Walker Department of Mechanical Engineering and is revolutionizing electronics and energy applications through cutting-edge atomistic modeling methods. His work delves into understanding, designing, and discovering materials, with a specific focus on electron transport, electrochemistry, catalysis, and 2D materials. Holding a B.S. degree from the University of Science and Technology of China (USTC) in 2008 and a Ph.D. from Rice University in 2014, Liu brings a wealth of expertise to his role. After postdoc studies at the National Renewable Energy Laboratory (NREL) and California Institute of Technology, he joined UT Austin in the Fall of 2017.
Saerom Yu and Zachary Levell are both students under Dr. Liu who joined his group in 2020 as Ph.D. candidates. Saerom's research focuses on atomic-scale reaction kinetics at electrochemical interfaces. She received her B.S. in Chemical Engineering at Soongsil University in South Korea and their M.S. in Energy at Texas A&M University in College Station, Texas. Zachary is currently studying novel materials for electrocatalysis using density functional theory. He obtained his B.S. in M.S. at the University of Urbana-Champaign. Xunhua Zhao was a postdoc from 2018-2022 at Texas Materials Institute but is currently a professor at Southeast University in China. Zhou was a visiting student in Liu’s group.
This work was recently published in Journal of the American Chemical Society by the American Chemical Society. This work is supported by Welch Foundation, National Science Foundation, and ACS Petroleum Research Fund. Please join us in congratulating them!


